Abstract
Introduction Acquired hemophilia (AH) is a rare disorder with an incidence of 1.48 per million per year and inhibitor to factor VIII (fVIII) is mostly implicated. The most common identifiable causes of acquired hemophilia A (AHA) include malignancies, pregnancy, and autoimmune disorders. AH following SARS Co-V2 vaccination has recently been reported. Here we provide the results of a comprehensive literature review of AH following SARS Co-V2 and influenza vaccinations.
Methods We searched PubMed, Embase and Scopus database for all relevant articles from database inception to July 1, 2022. Articles were included if the study described AH following vaccination. A total 1006 records were screened by title and abstracts out of which 21 full-text articles met eligibility criteria. Title and abstracts of all articles were retrieved using the search strategy were initially screened, reviewed, and verified independently by two authors (KP and SS), with disagreements mediated through discussion with a third review author (DG).
Most of the data included case reports except 3 case series and 2 letters. Additionally, Vaccine Adverse Event Reporting System (VAERS) database was also queried to assess for reports of hemophilia associated with receipt of vaccine till July 29, 2022. However these studies were not included in the analysis.
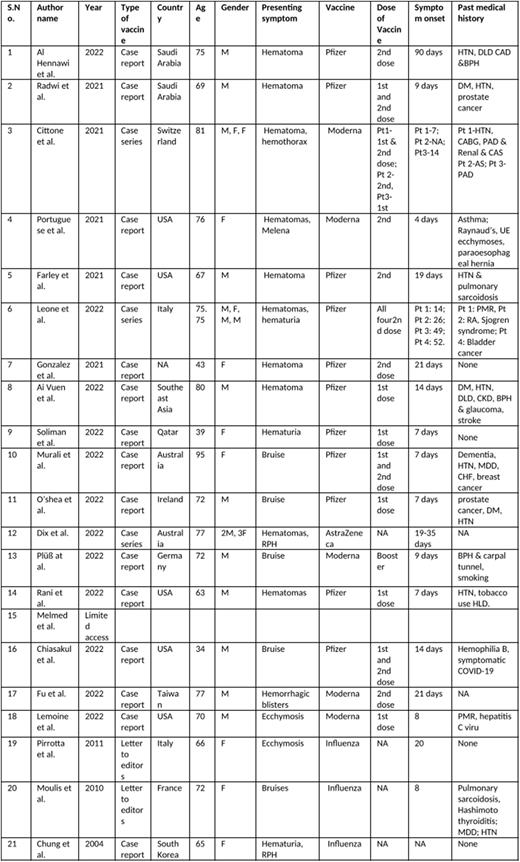
Results Out of 21 articles, 18 described AH following SARS Co-V2 vaccination and 3 AH following influenza vaccination with a total of 29 patients (Table 1). All patients developed factor VIII inhibitor except 1 case of factor IX inhibitor described by Chiasakul et al. The median age of AH following SARS Co-V2 vaccination was 68 years (SD 15.4 years) and 66 years (SD 3 years) following influenza vaccination. The complication has been reported more frequently in males (61%). Most patient developed cutaneous hematomas following COVID-19 vaccine but single cases of hemothorax, melena, knee hematoma and oropharyngeal bleeding was also described. Three patients developed hematuria, and 2 patients developed extensive retroperitoneal hemorrhage following vaccination. AH has been described following the Pfizer (53% of the cases we identified), Moderna (28% of the cases we identified), Astra Zeneca (19% of the cases we identified) vaccines. Within this small cohort, most patients (47%) developed AH following second dose of primary vaccination followed by AH developing after first dose (28%). Few patients developed AH following both doses of primary vaccination (19%) and 1 developed AH following booster dose. Only 1 case had history of COVID-19 infection. The average duration for onset of symptoms from SARS Co-V2 vaccination was 2 weeks 4 days and was 2 weeks following influenza vaccination. For inhibitor management, 11 patients received rituximab (RTX) with prednisone and 7 patients received cyclophosphamide with prednisone. Triplet therapy (steroids, RTX and cyclophosphamide) was used in 3 patients. Most patients developing AH following SARS Co-V2 vaccination improved except 2 deaths from abdominal bleeding and sepsis; 1 patient died from severe retroperitoneal bleeding after influenza vaccination.
We found 13 cases of AH and 2 cases of bleeding without diagnosis on VAERS database following COVID-19 vaccination and 2 cases developed factor IX inhibitor.
Conclusions Autoantibodies to FVIII (or FIX) can develop following SARS Co-V2 and influenza vaccines. The rate of this complication appears to very low, but it can lead to serious bleeding, with an average onset of symptoms 2 weeks from vaccine administration. We found more reports of males who developed AH after SARS Co-V2 vaccination, but neither the overall frequency nor the relative frequency (males vs. females) is known.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal